Hypochlorous Acid Formula: A Comprehensive Guide To Its Chemistry And Applications
Hypochlorous acid formula is a topic of immense importance in chemistry, healthcare, and environmental science. This compound, often represented by the chemical formula HOCl, plays a critical role in disinfection, water treatment, and even biological processes. As a weak acid, hypochlorous acid is highly effective in neutralizing pathogens while remaining safe for use in various applications. Understanding its formula, properties, and uses is essential for professionals and enthusiasts alike.
Hypochlorous acid is widely recognized for its antimicrobial properties. It is a naturally occurring compound in the human immune system, where it helps fight infections. Beyond its biological significance, it is also a key ingredient in household and industrial cleaning products. Its effectiveness, combined with its eco-friendly nature, has made it a preferred choice for disinfection in recent years.
In this article, we will explore the chemical structure of hypochlorous acid, its formula, and its applications in various fields. We will also discuss its safety, environmental impact, and how it compares to other disinfectants. By the end of this guide, you will have a comprehensive understanding of hypochlorous acid and its importance in modern science and industry.
Read also:What Is Doraemon The Timeless Robotic Cat From The Future
Table of Contents
- Chemical Structure of Hypochlorous Acid
- Understanding the Hypochlorous Acid Formula
- Key Properties of Hypochlorous Acid
- The Biological Role of Hypochlorous Acid
- Applications of Hypochlorous Acid
- Hypochlorous Acid vs. Other Disinfectants
- Safety and Environmental Impact
- How Hypochlorous Acid is Produced
- The Future of Hypochlorous Acid
- Conclusion and Call to Action
Chemical Structure of Hypochlorous Acid
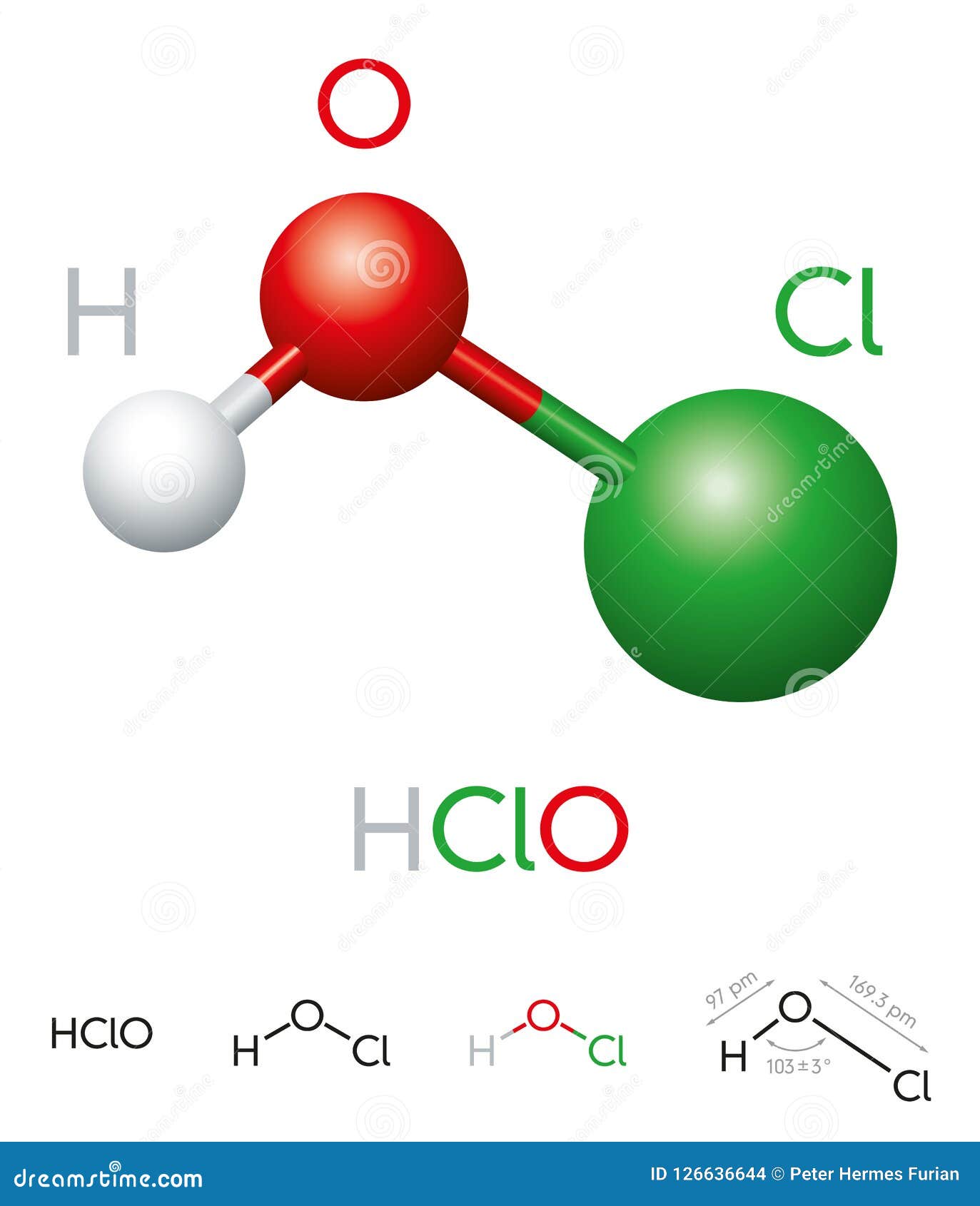
Hypochlorous acid is a simple molecule composed of three atoms: one hydrogen (H), one oxygen (O), and one chlorine (Cl). Its chemical formula, HOCl, reflects this composition. The structure of hypochlorous acid is characterized by a single bond between oxygen and hydrogen, with chlorine attached to the oxygen atom via a double bond. This arrangement gives hypochlorous acid its unique properties and reactivity.
Bonding and Molecular Geometry
- Hypochlorous acid has a bent molecular geometry due to the lone pairs of electrons on the oxygen atom.
- The bond angle between the hydrogen, oxygen, and chlorine atoms is approximately 103 degrees.
- This molecular geometry contributes to its polarity and ability to interact with other molecules.
Stability and Reactivity
Despite its simple structure, hypochlorous acid is highly reactive. It readily dissociates in water to form hypochlorite ions (OCl⁻) and hydrogen ions (H⁺). This dissociation is influenced by factors such as pH, temperature, and concentration. Understanding these properties is crucial for optimizing its use in various applications.
Understanding the Hypochlorous Acid Formula
The hypochlorous acid formula, HOCl, is a representation of its molecular composition. Each element in the formula plays a specific role in determining the compound's properties and behavior. Hydrogen provides the acidic nature, oxygen contributes to its oxidizing ability, and chlorine is responsible for its antimicrobial activity.
Significance of the Formula
- The formula indicates that hypochlorous acid is a weak acid, meaning it does not completely dissociate in water.
- Its oxidizing properties make it effective in neutralizing a wide range of pathogens, including bacteria, viruses, and fungi.
- The formula also highlights its simplicity, which contributes to its safety and eco-friendliness.
Variations of the Formula
In different contexts, hypochlorous acid may be represented by related formulas, such as NaOCl (sodium hypochlorite) or Ca(OCl)₂ (calcium hypochlorite). These compounds are precursors to hypochlorous acid and are often used in its production. Understanding these variations is essential for selecting the appropriate form for specific applications.
Key Properties of Hypochlorous Acid
Hypochlorous acid exhibits several key properties that make it a versatile and effective compound. These properties include its acidity, oxidizing ability, and antimicrobial efficacy. Let's explore each of these in detail.
Acidity
Hypochlorous acid is classified as a weak acid, with a pKa value of approximately 7.5. This means it partially dissociates in water, forming hypochlorite ions and hydrogen ions. Its weak acidic nature makes it less corrosive compared to stronger acids, such as hydrochloric acid.
Read also:Raymond Washington The Untold Story Of A Visionary Leader
Oxidizing Ability
One of the most notable properties of hypochlorous acid is its strong oxidizing ability. It can oxidize a wide range of organic and inorganic substances, making it effective in disinfection and bleaching processes. This property is also responsible for its antimicrobial activity.
Antimicrobial Efficacy
Hypochlorous acid is highly effective against a broad spectrum of microorganisms. It disrupts the cell walls and membranes of bacteria, viruses, and fungi, leading to their inactivation. This property has made it a popular choice for healthcare and food safety applications.
The Biological Role of Hypochlorous Acid
Hypochlorous acid plays a vital role in the human immune system. It is produced by neutrophils, a type of white blood cell, during the immune response. This natural production helps the body fight infections and maintain health.
Production in the Immune System
- Neutrophils produce hypochlorous acid by converting chloride ions (Cl⁻) into hypochlorite ions (OCl⁻) using the enzyme myeloperoxidase.
- This process occurs during the respiratory burst, a critical phase of the immune response.
- Hypochlorous acid helps neutralize pathogens by oxidizing their cellular components.
Biological Significance
The production of hypochlorous acid in the immune system underscores its importance in human health. Its ability to neutralize pathogens without causing significant harm to host tissues highlights its safety and effectiveness.
Applications of Hypochlorous Acid
Hypochlorous acid is used in a wide range of applications, from healthcare to environmental management. Its versatility, safety, and effectiveness make it a preferred choice for many industries.
Healthcare
- Hypochlorous acid is used as a disinfectant in hospitals and clinics to sterilize surfaces and equipment.
- It is also used in wound care to promote healing and prevent infections.
- Its non-toxic nature makes it safe for use on sensitive skin and mucous membranes.
Food Safety
In the food industry, hypochlorous acid is used to sanitize surfaces, equipment, and produce. It effectively eliminates pathogens without leaving harmful residues, making it ideal for food processing and packaging.
Water Treatment
Hypochlorous acid is widely used in water treatment to disinfect drinking water and wastewater. Its ability to neutralize pathogens while being environmentally friendly has made it a popular choice for municipal water systems.
Hypochlorous Acid vs. Other Disinfectants
When compared to other disinfectants, hypochlorous acid stands out for its safety, effectiveness, and eco-friendliness. Let's compare it to some commonly used disinfectants.
Comparison with Chlorine
While chlorine is a widely used disinfectant, it is more corrosive and can produce harmful byproducts. Hypochlorous acid, on the other hand, is less corrosive and does not produce significant byproducts, making it a safer alternative.
Comparison with Alcohol
Alcohol-based disinfectants are effective but can be flammable and drying to the skin. Hypochlorous acid is non-flammable and gentle on the skin, making it a better choice for frequent use.
Comparison with Hydrogen Peroxide
Hydrogen peroxide is another popular disinfectant, but it is less stable and can degrade quickly. Hypochlorous acid is more stable and retains its effectiveness for longer periods.
Safety and Environmental Impact
Hypochlorous acid is considered safe for use in various applications. Its non-toxic nature and minimal environmental impact make it a preferred choice for many industries.
Safety Considerations
- Hypochlorous acid is non-irritating to the skin and eyes, making it safe for use on sensitive surfaces.
- It does not produce harmful fumes or residues, reducing the risk of exposure to toxic substances.
- Its weak acidic nature minimizes the risk of corrosion and damage to materials.
Environmental Impact
Hypochlorous acid is environmentally friendly, as it breaks down into harmless substances such as water and salt. This makes it an ideal choice for applications where environmental sustainability is a priority.
How Hypochlorous Acid is Produced
Hypochlorous acid can be produced through various methods, including electrolysis and chemical synthesis. Let's explore these methods in detail.
Electrolysis
- Electrolysis involves passing an electric current through a solution of salt (NaCl) and water to produce hypochlorous acid.
- This method is widely used due to its simplicity and efficiency.
- It allows for the production of hypochlorous acid on-site, reducing the need for transportation and storage.
Chemical Synthesis
Chemical synthesis involves reacting chlorine gas with water to produce hypochlorous acid. While effective, this method requires careful handling of chlorine gas, which can be hazardous.
The Future of Hypochlorous Acid
The future of hypochlorous acid looks promising, with ongoing research and development aimed at expanding its applications. Innovations in production methods and formulations are expected to enhance its effectiveness and accessibility.
Emerging Applications
- Hypochlorous acid is being explored for use in agriculture to control plant pathogens and improve crop yields.
- It is also being investigated for use in air purification systems to reduce airborne pathogens.
- New formulations are being developed to extend its shelf life and improve its stability.
Research and Development
Researchers are studying the potential of hypochlorous acid in areas such as cancer treatment and biofilm eradication. These studies aim to unlock new possibilities for this versatile compound.
Conclusion and Call to Action
Hypochlorous acid, represented by the formula HOCl, is a remarkable compound with a wide range of applications. Its chemical structure, properties, and biological role make it an invaluable tool in healthcare, food safety, and environmental management. As research continues to uncover new uses and formulations, its importance is only expected to grow.
We hope this article has provided you with a comprehensive understanding of hypochlorous acid and its significance. If you found this guide helpful, please share it with others who may benefit from this information. Feel free to leave a comment or explore more articles on our site to deepen your knowledge. Together, let's stay informed and make the most of the incredible potential of hypochlorous acid.
Tauren Wells Ethnicity: Exploring The Roots Of A Multi-Talented Artist
Rebecca J OF: Unveiling The Rising Star's Journey, Achievements, And Influence
1965: The Year That Shaped The Modern World
HClO Hypochlorous Acid Molecule Model and Chemical Formula Stock Vector

MJ6001 Hypochlorous Acid bactericide RAYMOND