Molar Mass Of CoCl2: A Comprehensive Guide
Table of Contents
Understanding the molar mass of CoCl2 is crucial for students, researchers, and professionals in chemistry. Cobalt(II) chloride, commonly known as CoCl2, is a compound widely used in laboratories and industries. Its molar mass plays a key role in determining its chemical behavior and practical applications. By mastering the concept of molar mass, you can unlock the potential of this versatile compound in various scientific and industrial processes.
When we talk about the molar mass of CoCl2, we are referring to the mass of one mole of this compound. This value is essential for performing stoichiometric calculations, preparing solutions, and understanding reaction mechanisms. Whether you're a chemistry student or a professional in the field, knowing how to calculate and apply the molar mass of CoCl2 is a fundamental skill.
Read also:Doraemon Birth Date Unveiling The Origins Of The Beloved Robot Cat
In this article, we will delve into the concept of molar mass, explain how to calculate the molar mass of CoCl2, and explore its various applications. By the end of this guide, you'll have a comprehensive understanding of CoCl2 and its significance in chemistry. Let’s get started!
What is CoCl2?
Cobalt(II) chloride, or CoCl2, is an inorganic compound that appears as a blue crystalline solid in its anhydrous form and as a pink solid in its hydrated form (CoCl2·6H2O). This compound is highly soluble in water and alcohol, making it a popular choice in laboratories for various chemical reactions and experiments.
CoCl2 is widely used as a catalyst, a desiccant, and in the production of pigments and dyes. Its ability to absorb moisture makes it an excellent drying agent, while its vibrant color changes serve as an indicator for water presence in certain chemical processes.
Here are some key facts about CoCl2:
- Chemical formula: CoCl2
- Molar mass: 129.839 g/mol (anhydrous)
- Appearance: Blue crystalline solid (anhydrous), pink solid (hydrated)
- Solubility: Highly soluble in water and ethanol
- Uses: Catalyst, desiccant, pigment production
Understanding Molar Mass
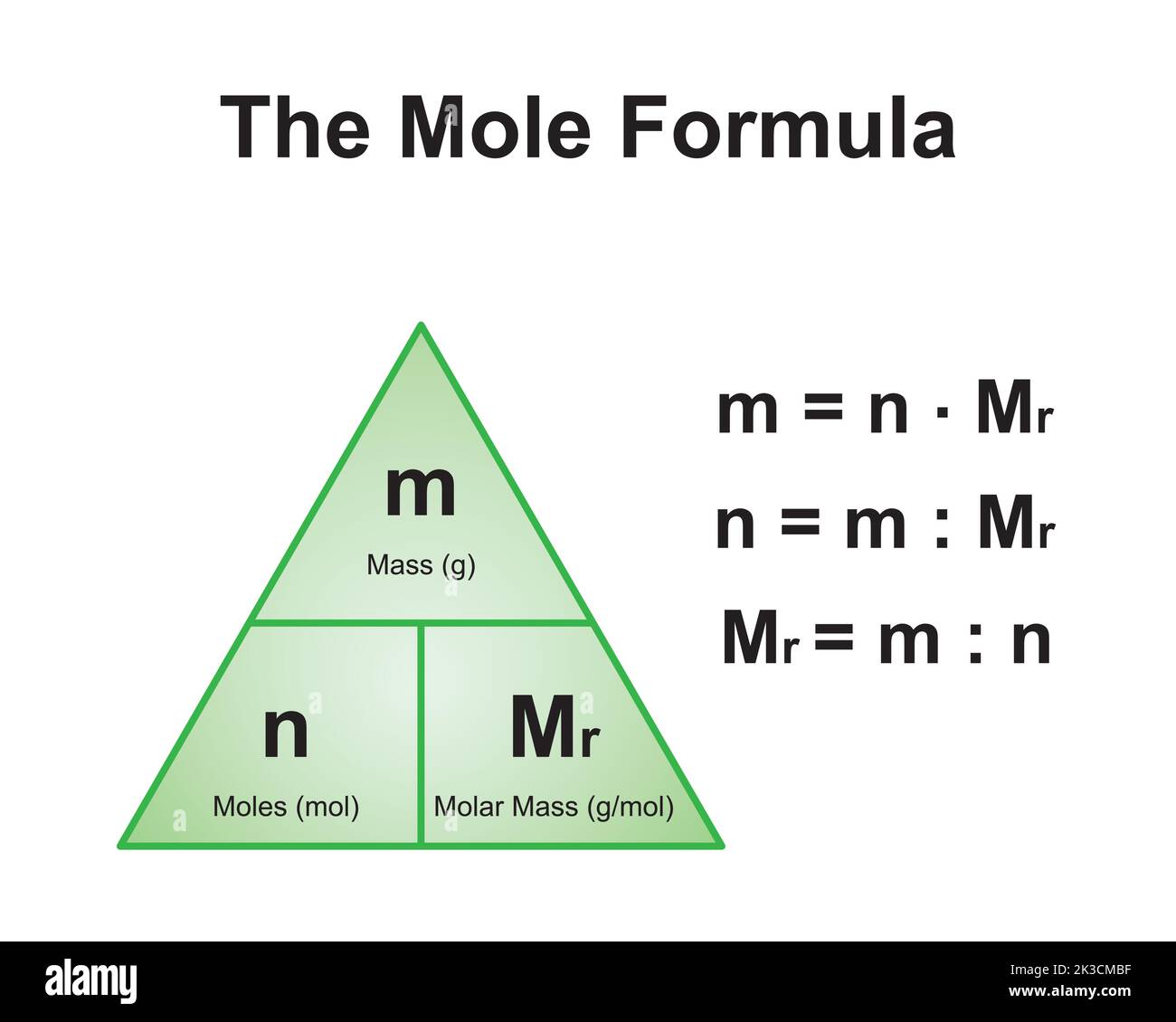
Molar mass is a fundamental concept in chemistry that refers to the mass of one mole of a substance. It is expressed in grams per mole (g/mol) and is calculated by summing the atomic masses of all the atoms in a chemical formula. For example, the molar mass of water (H2O) is calculated by adding the atomic masses of two hydrogen atoms and one oxygen atom.
The molar mass of a compound is essential for various applications, such as:
Read also:Doraemon 2026 Movie A Glimpse Into The Future Of Anime Entertainment
- Stoichiometric calculations in chemical reactions
- Preparing solutions of specific concentrations
- Understanding the behavior of substances in reactions
For CoCl2, the molar mass is determined by adding the atomic masses of cobalt (Co) and two chlorine (Cl) atoms. This calculation provides the foundation for understanding the compound's properties and applications.
How to Calculate Molar Mass of CoCl2
Calculating the molar mass of CoCl2 is a straightforward process that involves summing the atomic masses of its constituent elements. Here’s a step-by-step guide:
- Identify the elements in the compound: Cobalt (Co) and Chlorine (Cl).
- Find the atomic masses of these elements from the periodic table:
- Cobalt (Co): 58.933 g/mol
- Chlorine (Cl): 35.453 g/mol
- Multiply the atomic mass of chlorine by 2 (since there are two chlorine atoms in CoCl2).
- Add the atomic masses together:
- Molar mass of CoCl2 = 58.933 + (35.453 × 2) = 129.839 g/mol
By following these steps, you can determine the molar mass of CoCl2 accurately. This value is critical for performing precise chemical calculations and ensuring the success of experiments.
Applications of CoCl2
CoCl2 has a wide range of applications in various fields, thanks to its unique properties. Below are some of the most notable uses:
- Catalyst: CoCl2 is used as a catalyst in organic synthesis, particularly in hydroformylation reactions.
- Desiccant: Its ability to absorb moisture makes it an effective drying agent in laboratories and industries.
- Pigment Production: CoCl2 is used in the production of pigments and dyes, providing vibrant colors.
- Humidity Indicator: The color change of CoCl2 from blue to pink in the presence of water makes it an excellent humidity indicator.
These applications highlight the versatility of CoCl2 and its importance in both academic and industrial settings.
Chemical Properties of CoCl2
CoCl2 exhibits several unique chemical properties that contribute to its widespread use. Understanding these properties is essential for utilizing the compound effectively:
- Solubility: Highly soluble in water and ethanol, making it easy to prepare solutions.
- Hygroscopic Nature: CoCl2 readily absorbs moisture from the air, which is why it is used as a desiccant.
- Color Change: The compound changes color from blue (anhydrous) to pink (hydrated) when exposed to water.
- Reactivity: CoCl2 reacts with various substances, making it a valuable catalyst in chemical reactions.
These properties make CoCl2 a versatile compound with applications in diverse fields, from chemistry labs to industrial processes.
Safety Precautions
While CoCl2 is a valuable compound, it is important to handle it with care due to its potential hazards. Here are some safety precautions to keep in mind:
- Toxicity: CoCl2 is toxic if ingested or inhaled. Always use protective equipment such as gloves and goggles when handling it.
- Moisture Sensitivity: Store CoCl2 in a dry environment to prevent it from absorbing moisture and changing its properties.
- Disposal: Dispose of CoCl2 waste according to local regulations to prevent environmental contamination.
- Ventilation: Work in a well-ventilated area to avoid inhaling fumes or dust from CoCl2.
By following these safety guidelines, you can ensure the safe and effective use of CoCl2 in your experiments and applications.
CoCl2 in Industry
CoCl2 plays a significant role in various industrial processes, contributing to the production of essential materials and chemicals. Some of its key industrial applications include:
- Polymer Production: Used as a catalyst in the production of polymers and plastics.
- Petroleum Refining: Acts as a catalyst in hydroformylation reactions during petroleum refining.
- Textile Industry: Used in dyeing and printing processes to produce vibrant colors.
- Electronics: Applied in the production of certain electronic components due to its conductive properties.
These industrial applications highlight the importance of CoCl2 in modern manufacturing and technology.
Frequently Asked Questions
What is the molar mass of CoCl2?
The molar mass of CoCl2 is 129.839 g/mol. This value is calculated by summing the atomic masses of cobalt and two chlorine atoms.
Why is molar mass important?
Molar mass is crucial for performing stoichiometric calculations, preparing solutions, and understanding the behavior of substances in chemical reactions.
Is CoCl2 toxic?
Yes, CoCl2 is toxic if ingested or inhaled. Proper safety precautions should be taken when handling this compound.
Conclusion
In this article, we have explored the concept of molar mass and its application to cobalt(II) chloride (CoCl2). We learned how to calculate the molar mass of CoCl2, discussed its chemical properties, and examined its various applications in laboratories and industries. Understanding the molar mass of CoCl2 is essential for performing accurate chemical calculations and ensuring the success of experiments and industrial processes.
We also highlighted the importance of safety precautions when handling CoCl2 due to its potential hazards. By following these guidelines, you can use CoCl2 safely and effectively in your work.
If you found this article helpful, feel free to share it with others or leave a comment below. For more informative articles on chemistry and related topics, explore our website and expand your knowledge!
Discover The Hidden Treasures Of Local Gems Mexico: A Comprehensive Guide
The Inspiring Journey Of Figure Skater Tara Lipinski: Achievements, Legacy, And Life Lessons
INFP Perfect Match: Discovering The Ideal Relationship For The Idealist Personality
[Solved] What is the molar mass of CoCl2. The molar mass of CoCle is
Scientific Designing of The Mole Formula Triangle. Relationship Between