What Is The Chemical Formula For Hypochlorous Acid? A Comprehensive Guide
Hypochlorous acid is a fascinating compound with significant applications in various fields, including healthcare, sanitation, and water treatment. Understanding its chemical formula and properties is essential for anyone looking to explore its uses and benefits. This article dives deep into the science behind hypochlorous acid, its chemical composition, and its wide-ranging applications.
Hypochlorous acid, often abbreviated as HOCl, is a weak acid that plays a crucial role in disinfection and cleaning processes. It is naturally produced by the human immune system to fight infections and is widely used in industrial and household cleaning products. Its effectiveness as a disinfectant and its safety for human use make it a highly sought-after compound.
In this article, we will explore the chemical formula of hypochlorous acid, its properties, and its applications in various industries. By the end of this guide, you will have a comprehensive understanding of this remarkable compound and how it impacts our daily lives. Let’s begin by breaking down its chemical composition.
Read also:Dekisugi Unveiling The Charismatic Protagonist Of Doraemon
Table of Contents
- The Chemical Formula of Hypochlorous Acid
- Chemical Properties of Hypochlorous Acid
- How Hypochlorous Acid is Formed
- Applications of Hypochlorous Acid
- Hypochlorous Acid in Healthcare
- Hypochlorous Acid in Water Treatment
- Safety and Handling of Hypochlorous Acid
- Environmental Impact of Hypochlorous Acid
- Comparison with Other Disinfectants
- Conclusion and Call to Action
The Chemical Formula of Hypochlorous Acid
Hypochlorous acid has the chemical formula HOCl. It consists of one hydrogen (H) atom, one oxygen (O) atom, and one chlorine (Cl) atom. This simple yet powerful molecule is responsible for its disinfectant properties. The chemical formula indicates that hypochlorous acid is a weak acid, meaning it does not completely dissociate in water.
When dissolved in water, hypochlorous acid partially dissociates into hypochlorite ions (OCl⁻) and hydrogen ions (H⁺). This equilibrium is crucial for its effectiveness as a disinfectant. The balance between HOCl and OCl⁻ determines its potency in killing bacteria, viruses, and other pathogens.
Understanding the Structure of HOCl
The molecular structure of hypochlorous acid is linear, with the chlorine atom bonded to the oxygen atom and the hydrogen atom attached to the oxygen. This arrangement allows hypochlorous acid to act as an oxidizing agent, which is key to its disinfectant properties.
Chemical Properties of Hypochlorous Acid
Hypochlorous acid is a weak acid with a pKa value of approximately 7.5. This means that at a neutral pH of 7, about half of the hypochlorous acid molecules exist in their undissociated form (HOCl), while the other half dissociate into hypochlorite ions (OCl⁻). The pH of the solution plays a significant role in determining the effectiveness of hypochlorous acid as a disinfectant.
Some key chemical properties of hypochlorous acid include:
- It is a strong oxidizing agent.
- It is unstable and decomposes quickly in sunlight or at high temperatures.
- It is effective at low concentrations, making it safe for use in various applications.
Oxidizing Power of Hypochlorous Acid
The oxidizing power of hypochlorous acid makes it highly effective in breaking down the cell walls of microorganisms. This property is what allows it to kill bacteria, viruses, and fungi. Unlike other disinfectants, hypochlorous acid is non-toxic to humans and animals when used in appropriate concentrations.
Read also:Orny Adams Age A Comprehensive Guide To The Comedians Life And Career
How Hypochlorous Acid is Formed
Hypochlorous acid can be formed through a simple chemical reaction between chlorine gas (Cl₂) and water (H₂O). The reaction is as follows:
Cl₂ + H₂O ⇌ HOCl + HCl
This reaction produces hypochlorous acid and hydrochloric acid (HCl). In industrial settings, hypochlorous acid is often generated using electrolysis of a saltwater solution, which is a more controlled and efficient method.
Electrolysis Process
During electrolysis, a saltwater solution (sodium chloride dissolved in water) is passed through an electrolytic cell. This process generates hypochlorous acid, sodium hydroxide, and hydrogen gas. The resulting solution contains hypochlorous acid, which can be used for disinfection purposes.
Applications of Hypochlorous Acid
Hypochlorous acid is widely used in various industries due to its effectiveness as a disinfectant and its safety for human use. Below are some of the most common applications:
- Healthcare: Used for wound cleaning and surface disinfection.
- Water Treatment: Effective in purifying drinking water and treating wastewater.
- Food Industry: Used to sanitize food processing equipment and surfaces.
- Household Cleaning: Found in many household cleaning products due to its non-toxic nature.
Hypochlorous Acid in the Food Industry
In the food industry, hypochlorous acid is used to sanitize equipment, surfaces, and even fresh produce. Its ability to kill pathogens without leaving harmful residues makes it an ideal choice for food safety applications.
Hypochlorous Acid in Healthcare
Hypochlorous acid has gained significant attention in the healthcare industry due to its effectiveness in wound care and surface disinfection. It is used in hospitals and clinics to clean and disinfect surfaces, medical instruments, and even patient wounds.
One of the key advantages of hypochlorous acid in healthcare is its non-toxic nature. Unlike other disinfectants, it does not cause skin irritation or respiratory issues, making it safe for use around patients and healthcare workers.
Wound Care with Hypochlorous Acid
Hypochlorous acid is particularly effective in wound care because it can kill bacteria and other pathogens without damaging healthy tissue. This makes it an excellent choice for treating chronic wounds, burns, and surgical incisions.
Hypochlorous Acid in Water Treatment
Hypochlorous acid plays a vital role in water treatment processes. It is used to purify drinking water, disinfect swimming pools, and treat wastewater. Its ability to kill bacteria, viruses, and other pathogens makes it an essential component of water sanitation systems.
In drinking water treatment, hypochlorous acid is added to water to eliminate harmful microorganisms. It is also used in swimming pools to maintain water hygiene and prevent the spread of waterborne diseases.
Advantages of Using Hypochlorous Acid in Water Treatment
Some of the advantages of using hypochlorous acid in water treatment include:
- Highly effective at low concentrations.
- Safe for human consumption when used in appropriate amounts.
- Environmentally friendly compared to other disinfectants.
Safety and Handling of Hypochlorous Acid
While hypochlorous acid is generally safe for use, it is important to handle it with care to avoid any potential risks. Below are some safety guidelines for handling hypochlorous acid:
- Store in a cool, dark place to prevent decomposition.
- Avoid exposure to sunlight or high temperatures.
- Use appropriate personal protective equipment (PPE) when handling concentrated solutions.
Decomposition of Hypochlorous Acid
Hypochlorous acid is unstable and can decompose into oxygen and hydrochloric acid when exposed to light or heat. This decomposition can reduce its effectiveness as a disinfectant, so proper storage is essential.
Environmental Impact of Hypochlorous Acid
Hypochlorous acid is considered environmentally friendly compared to other disinfectants. It breaks down into harmless substances like water, oxygen, and salt, leaving no toxic residues behind. This makes it a preferred choice for applications where environmental impact is a concern.
Biodegradability of Hypochlorous Acid
One of the key environmental benefits of hypochlorous acid is its biodegradability. It does not persist in the environment and does not contribute to pollution, making it a sustainable option for disinfection and cleaning.
Comparison with Other Disinfectants
Hypochlorous acid is often compared to other disinfectants like bleach (sodium hypochlorite) and hydrogen peroxide. While all these compounds are effective at killing pathogens, hypochlorous acid stands out due to its safety and environmental friendliness.
Below is a comparison of hypochlorous acid with other common disinfectants:
| Disinfectant | Effectiveness | Safety | Environmental Impact |
|---|---|---|---|
| Hypochlorous Acid | High | Safe for humans and animals | Biodegradable, no toxic residues |
| Bleach (Sodium Hypochlorite) | High | Toxic if ingested or inhaled | Potential environmental harm |
| Hydrogen Peroxide | Moderate | Safe in low concentrations | Breaks down into water and oxygen |
Why Choose Hypochlorous Acid?
Hypochlorous acid is the ideal choice for applications where safety and environmental impact are priorities. Its effectiveness, combined with its non-toxic nature, makes it a superior alternative to traditional disinfectants.
Conclusion and Call to Action
Hypochlorous acid, with the chemical formula HOCl, is a remarkable compound with a wide range of applications. Its effectiveness as a disinfectant, combined with its safety for human use and minimal environmental impact, makes it an invaluable tool in healthcare, water treatment, and beyond.
If you found this article informative, please consider sharing it with others who might benefit from learning about hypochlorous acid. Leave a comment below to share your thoughts or ask any questions you may have. For more in-depth articles on chemistry and science, explore our website and discover the fascinating world of molecules and compounds.
Doraemon Start Date: Exploring The Origins And Legacy Of A Timeless Anime
Raymond Washington: The Untold Story Of A Visionary Leader
Ratatouille Cast Brother: Exploring The Talented Voices Behind The Iconic Characters
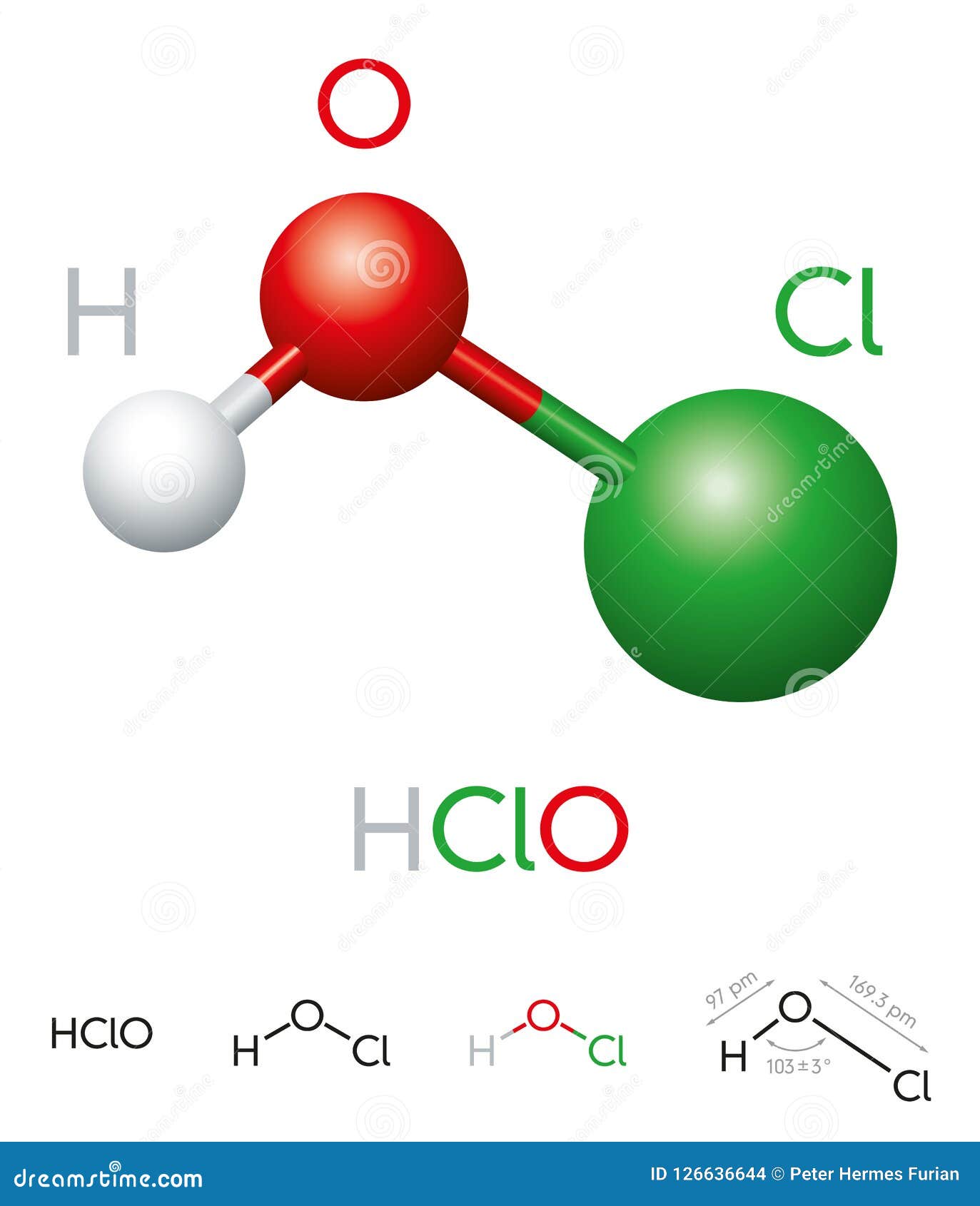
HClO Hypochlorous Acid Molecule Model and Chemical Formula Stock Vector

Hypochlorous Acid Hclo Molecule Chemical Structure Stock Illustration